In October, the Tufts Center for the Study of Drug Development published "Manufacturing Strategy for Diverse Biologic Pipelines of the Future." Abdullah Baaj, MD, PharmD; Parrish Galliher, MS; and Kenneth Kaitin, PhD based their work on a roundtable discussion two years previous that included senior managers from biologics development companies and officials from the U.S. Department of Defense and the U.S. Department of Health and Human Services Assistant Secretary for Preparedness and Response. (A follow-up workshop is planned in February.) Among the paper’s themes:
- Biologics makers are increasingly turning to manufacturing as a way to contain costs while increasing product quality and adapting to market demands.
- Continuous and real-time manufacturing can improve the government’s emergency preparedness to respond to real crises.
- Globalization and manufacturing decentralization have created significant investment opportunities for biologics manufacturing in emerging and developing markets.
Dig further into the 30-page document and you’ll find detailed schematics and notes from experts at such companies as M + W Group and GE Healthcare. You’ll also find compelling ideas complementary to those of the recently published white paper from the Industrial Asset Management Council and the Society of Industrial and Office Realtors, Rx for Change: The Flexible Biopharma Facility of the Future.

The white paper’s lead author is Dr. Abdullah Baaj, a Boston native and founder and CEO of Boston Oncology, a Cambridge, Massachusetts–based company with an affiliate partner — Tadawy, Bio-Medical Co., based in Riyadh, Saudi Arabia. The company focuses on in-licensing approved generic small-molecule products and co-developing and commercializing biosimilar products. Then it takes them on the road … a very specific road.
"Through our facilities in developing and emerging nations we locally-register, manufacture and market the specialty pharmaceuticals," the company explains. "We aim to invest in the development of emerging markets throughout the Middle East, North Africa, South America and Southeast Asia. Currently, we have operations in the Arabian Gulf and are targeting projects in Argentina, Malaysia and Turkey."
Could that list grow? The firm even offers a list of qualifying criteria:
"Nations ideally suited for development offer:
- A large population base with a strong growth rate
- A strong GDP with significant health care expenditures
- Supportive institutions including:
- established and expanding hospitals
- payers expanding insurance coverage
- increasing government promotion of joint purchasing, consumption of generics and local production of pharmaceuticals
- A scarcity of quality generic and/or locally manufactured pharmaceutical products
- The existence of several high-profile champions who want to be part of the pharmaceutical transformation.
At the company’s heart is, well, a heart, seeking to find ways for collaborating parties to exploit market opportunity without exploiting individuals. The large-scale, centralized supply chains of big pharma are fine for North America and Europe, but don’t really reach developing nations effectively. Boston Oncology’s niche is "setting up local manufacturing centers that feature cutting-edge single-use technologies that enable high-quality, small-batch production."
"Traditionally, developing nations have imported specialty drugs, like oncology treatments, in limited supply and at great cost from external manufacturers, to the detriment of local citizens and economy," the firm’s website explains. "We aim to provide a better model for those markets ready to invest in their own development. We do not simply sell pharmaceuticals. Rather, like the central atom in a molecule, we unite parties from three communities — in medicine, research and technology, and business — who share a commitment to the prosperity of the developing world."
"Twenty-one countries are considered by IMS to be ’emerging’ within the pharmaceutical world," noted Fortune in a 2015 article on "pharmerging" nations. "This category is defined as countries that are expected to see more than $1 billion in absolute spending growth from 2014 to 2018 and which currently have GDP per capita of less than $25,000."
After first talking to Abaaj as he waited in the Copenhagen airport to board a 10-hour flight to the Middle East, I caught up with him earlier this month in Dubai, where he briefly described his firm’s own approach to site selection:
"At a high level, there is no criteria that is special compared to any other investment opportunity," he says. "We evaluate ROI. That said, we care greatly about population size — we have our own index for minimal size acceptable for investment; government investment in local health care; and geography-based asymmetric advantages."
Obstacles can include regulatory challenges and that universal challenge: finding enough skilled labor.
"Development and manufacturing of biologic molecules are complex activities," Baaj explains. "All regulators are reluctant to be pioneers in an understudied space."
What about Big Pharma? Friend, foe or somewhere in between?
"Seemingly, we are competitors," Baaj answers. "Realistically, however, we move in parallel. Our strategy is to be nimble. Big Pharma are giants and move as such."
To download a copy of the Tufts white paper, visit http://csdd.tufts.edu/reports/white_papers. With the permission of Baaj and his co-authors, below is the paper’s introduction:
The biotherapeutics industry is expanding rapidly across the globe, spurred by blockbuster biologics falling off the patent cliff, an uptick in orphan drugs, and the advent of biosimilars and personalized medicine. Where once biologics and orphan drugs were niche markets with few therapies in each space, more than 1,000 large molecules are in development today, and 250 orphan drugs have been approved in the U.S. Just nine years ago, only 11 drugs fit the definition of personalized medicine. By 2015, that number had grown 10-fold. It is expected that, within a few years, personalized medicines will have multiplied 100-fold.
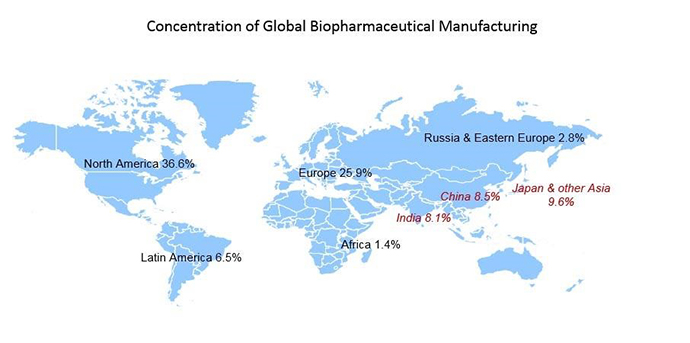
At the same time, the range of biologics is expanding at unprecedented levels. Once the domain of vaccines and simple proteins, biologics now encompass antibodies, immunotherapies, stem cell and tissue-based products, and even nutraceuticals. Meanwhile, the biologics industry is facing epic globalization and decentralization of manufacturing processes. Just 20 years ago, biologics manufacturing was confined to a handful of plants in the U.S. and Europe. Today, the U.S. accounts for only 37 percent of the world’s biopharmaceutical manufacturing. Asia has slightly outpaced Europe with more than 26 percent of global drug manufacturing, and other bio-clusters are springing up in Latin America, Eastern Europe and Africa.
In the midst of such shifts, biologics have continued an upward trend in pricing, with some touting six-figure treatment costs in the U.S. Given the increased use of the drugs and growing competition, pressure is mounting to bring prices down. Consequently, biologics makers are looking more and more to manufacturing as the strategic driver of the commercial success of their products. They see manufacturing as a way to contain costs while increasing product quality and adapting to the demands of the 21st century biologics market. In the manufacturing plants of the future, flexibility will be key as drugmakers produce smaller runs of a greater variety of products in each facility.
To respond to the changing marketplace, the biologics industry will have to be as innovative with manufacturing technologies and processes as it is with the drugs it produces. The future of the industry rests on its ability to develop more cost-effective manufacturing technologies, tools and processes that can meet shifting demands of scale and product diversity while improving quality. New benchmarks, standards and best practices are needed to make manufacturing as efficient and flexible as possible to keep up with the dictates of a more diverse and global biologics pipeline.