Rock Health — the first venture fund dedicated to digital health — is a fitting prism for viewing the current and future world of digital health technologies, and how they’re navigating their own paths through the multinational life sciences and medical device ecosystem.
As the COVID-19 pandemic has unfolded, they navigated a path to immediate action — and job creation.
With early partnerships signed with such major companies as Humana and Walmart, telehealth company Doctor on Demand was already in great demand itself. COVID-19 made it more so, as the company created an online assessment tool for the virus based on CDC guidelines, and had its doctors available 24/7 over video to evaluate individual risk and determine appropriate next steps. The activity sparked a new wave of hiring corresponding to the waves of the pandemic.

“The COVID-19 crisis has created a sea change in how patients and healthcare organizations view the importance of telehealth and their receptivity to utilizing virtual care platforms.”
“Virtual care serves a vital public health role during the COVID-19 pandemic,” explains Robin Glass, president of Doctor on Demand, in an interview. “Our team mobilized quickly and engaged directly with the CDC to help with preparation, surveillance, triage and treatment of patients with suspected COVID-19. We have seen a significant increase in demand as government and health officials point Americans to telehealth as a frontline source of care to stay safe, and prevent the spread of infection. In March, we saw 110% more demand than we had seen the month prior when the outbreak had just begun.”
It isn’t just pandemic-induced calls coming in, she says, as patients have turned to virtual care for acute and chronic conditions as they opt to stay home instead of seeking in-person care. “We have also seen an increase in patient visits for our mental health services,” Glass says.
Asked how much hiring the company has done, Glass says, “In response to the rapid increases in demand, we brought on hundreds of additional licensed physicians, psychologists and psychiatrists to provide care.” Other telehealth firms have done likewise, with 98point6 announcing plans to triple its physician workforce over one month and PlushCare planning to increase hiring by between 50% and 100%. For them it’s a continuation of a trend that since 2015 has seen live video telemedicine use expand by 4.5 times, according to a consumer survey Rock Health conducted last year.
Where do Doctor on Demand’s doctors work? Just like us, their status is WFH: “As a virtual care company delivering care in all 50 states, our nationwide practice of physicians work from their home offices,” says Glass.


The company itself was founded in San Francisco in 2012, and maintains a workforce of 205 employees at its offices in that city, Minneapolis and D.C., as well as in-home offices. In other words, as companies during this crisis have witnessed how working from home and remotely can still be productive, Doctor on Demand has been at it a while already, and thus offers a model for those considering more WFH in their future workspace allocation plans.
“Our culture is video-based through and through,” says Glass. “Similar to how our technology puts patients face-to-face with doctors, our teams are accustomed to jumping onto video calls throughout the day.”
So the company whose physicians can work from anywhere can, literally, expand anywhere it wants, one qualified individual at a time.
“At Doctor On Demand, we provide care to patients in all 50 states, 24/7/365,” Glass explains. “We have seen interest nationwide from health plans, employers and government programs who would like to provide Doctor On Demand to their populations. During the COVID-19 crisis, we’ve collaborated closely with local provider organizations as well as public health officials to reduce the risk of exposure to patients and healthcare workers, and to ensure that patients are getting the appropriate in-person care.
“This pandemic has encouraged many people — both patients and healthcare stakeholders — to think about the role of digital health differently,” she says. “In the past, digital health solutions such as telehealth were seen as niche players in the overall healthcare ecosystem. The COVID-19 crisis has created a sea change in how patients and healthcare organizations view the importance of telehealth and their receptivity to utilizing virtual care platforms.”
Tech is Already Endemic
In many ways, health tech has gone from a novelty to part of the fabric of the life sciences sector. The pandemic is simply accelerating that trajectory.
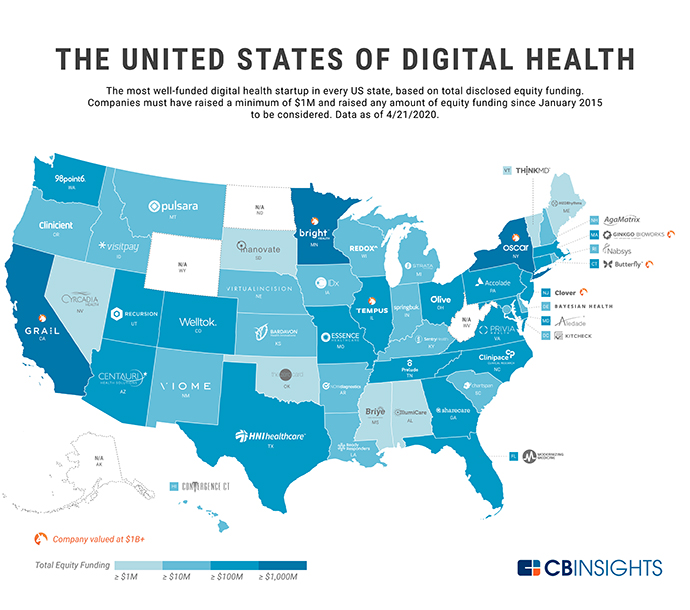
An April 2020 report from CB Insights that highlighted the top-funded digital health startup in every U.S. state noted that seed/angel have dropped to 30% of deal share in 2020 year-to-date (YTD), after accounting for more than half of deals in 2015. But at the same time, digital health startups raised over $17 billion across more than 1,700 deals in 2019. “Now, amid the COVID-19 pandemic, many of these startups are receiving more attention than ever,” CB Insights noted. “In 2020 YTD, the states that have seen the most equity deals to digital health startups are, unsurprisingly, California, New York, and Massachusetts — but investors have bet on the sector across the country.”
Among the leaders nationwide: cancer-focused molecular and clinical data startup Tempus (Illinois), emerging health insurer Bright Health (Minnesota), and insurtech unicorn Clover Health (New Jersey). Among other insights:
- The most well-funded U.S. digital health startup is California-based early cancer detection startup Grail, valued at $3.2 billion with $1.7 billion in total disclosed equity funding. Grail is followed by New York-based tech-focused health insurer Oscar Health ($1.3 billion in total disclosed funding), Bright Health ($1.1 billion), Clover Health ($838 million) and Massachusetts-based DNA startup Ginkgo BioWorks ($726 million).
- Health insurance, genomics and microbe engineering (Gingko and another firm Zymergen are examples) are common focus areas for these startups. So is a fast-growing digital healthcare space that encompasses “everything from disease diagnostics and management, to health insurance built on tech infrastructure, to AI tools for drug discovery.”
Companies across Rock Health’s portfolio are not only active in these areas, but active today in fighting the pandemic. As noted in a recent Rock Health blog: AgileMD’s clinical decision support tools are helping leading hospitals automate screening and manage pathways for COVID-19 cases free of charge. Ambient Clinical Analytics is supporting hospitals in managing the surge in demand for ICU beds. Augmedix is expanding its capabilities to support clinicians who are on the front lines with its hands-free, remote documentation solution that helps clinicians remain in their personal protective equipment and continue treating patients while minimizing exposure risk. And that’s just the “A”s.
ClinCapture is giving its electronic data capture (EDC) system at no cost to biopharma and diagnostic companies that are running clinical trials for COVID-19. Elemental Machines is providing two months of free lab monitoring for biotech and research facilities, where lab scientists cannot simply “work from home.” Kit Check is helping hospitals keep track of inventory, location, and usage of medications — including those being explored as possible treatments for COVID-19, such as hydroxychloroquine. “As some hospitals see hoarding or reckless prescriptions of these drugs,” Rock Health explains, “Kit Check’s medication tracking and diversion risk assessment solutions empower hospital pharmacists and staff to make sure these potentially life-saving medications get to the patients who really need them.”
Evidation launched a nationwide study to track the attitudes, behaviors, and overall health of Americans during the course of the pandemic. Over 185,000 people from all 50 states and the District of Columbia agreed to participate as of mid-April, and were recruited in less than four weeks from the nearly 4 million people who use Evidation’s Achievement app, creating what Evidation calls “the largest, most diverse virtual research site in the U.S.”
The study follows another use of Evidation tech by Johnson & Johnson’s Janssen Pharmaceutical Companies, which in February, in collaboration with Apple, opened enrollment for a study designed to explore if the Heartline Study app on iPhone and heart health features on Apple Watch can improve health outcomes, including reducing the risk of stroke, with earlier detection of atrial fibrillation (AFib). Through the app-based approach, the study will enable participants to engage in the study remotely, right from their iPhone and in some cases an Apple Watch, rather than travel to a clinical trial site, thus potentially saving time and cost.
The study “has the potential to fundamentally change our understanding of how digital health tools, like the ECG app and irregular rhythm notification feature on Apple Watch, could lead to earlier detection of AFib, helping patients understand and directly engage in their heart health, prompting potentially life-saving conversations with their doctors, and improving health outcomes,” said Dr. C. Michael Gibson, Professor of Medicine, Harvard Medical School, and CEO of the Baim Institute.
Although digital health saw a record $3.1 billion in funding in Q1 2020, the pandemic will rattle this sector like every other. A survey of 12 healthcare investors (including itself) that Rock Health conducted in March found 92% of investors agree digital health companies anticipating a 2020 IPO will not go public as planned.
“Yet two important factors may buffer a contraction in digital health venture funding,” Rock Health noted. One is that venture agreements make it hard for limited partners (the capital suppliers) to walk away. The other is that “digital health startups are, in some cases, uniquely positioned to play both an outsized role in ameliorating the immediate effects of the crisis and in driving sustained, positive changes in its aftermath. While other industries (or other segments within healthcare) must simply cope, some digital health companies are in the driver’s seat.”
“The most significant problem facing healthcare is a historic mismatch between supply and demand,” stated Rock Health President Tom Cassels. “This was true before the onset of the pandemic and it will remain true after COVID-19 peaks. The human capital (MDs and nurses) mismatch is most acute today, hence the lift-off for telemedicine and remote patient monitoring solutions. If there is one thing I am confident in predicting, it is that overcoming COVID-19 will reinforce that healthcare cannot go back to a time when virtual or automated care was not normal operating procedure.”
Quebec Tech
In a 2018 interview, Steven Arless, an entrepreneur in residence with an extensive medtech resume, said one of that province’s primary industry sectors was a big reason behind Quebec’s healthtech success and crucial to one of his big scores: the C$400-million sale of CryoCath to Medtronic in 2008.
“Bottom line, what made me successful was the great resource pool here from the aerospace sector,” he told me. “In every complete medtech development, you need to have experienced engineers trained to work in a multi-disciplined environment that is highly regulated. Aerospace is highly regulated by the FAA. And it’s similar in medtech. We ended up employing 300 people. Thank god for Quebec’s aerospace talent pool. It’s still a great place because of that.”
Arless is entrepreneur in residence at Centech, a tech company accelerator affiliated with the École de Technologie Supérieure in Montreal whose roster of companies is around 25% medtech. Last year the accelerator inaugurated 10 new corporate space for open innovation, with immediate tenants including CAE, Siemens and Thales.
One of the firms Arless founded is SoundBite Medical Solutions, which is developing and commercializing proprietary wire-based device and generator console that delivers safe shockwave energy within the cardiovascular system to treat chronic total occlusions (CTOs). The firm, located at Technoparc Montreal in Montreal’s growing West Island area near Montreal-Trudeau InternationalAirport, that year celebrated a $20-million round of Series A financing as well as a crucial certification milestone, for technology first developed out of the University of Sherbrooke two hours to the east.

“Bioengineers are pioneering the development of cutting-edge, cost-effective, mobile and point-of-care technologies.”
In September 2019 SoundBite entered into an $8 million loan agreement with Investissement Québec as part of the province’s Biomed Propulsion Program. And in February of this year, the firm received FDA IDE (Investigational Device Exemption) approval to start a study with the device the loan has helped to develop.
“The cost of developing technology in Quebec versus Boston or California is one-third the cost after tax,” Arless told me in 2018. “I know a number of VCs in New York, and all they do is look for medtech opportunities in Canada because of that cost competitiveness. The other reason why Quebec will be a great spot for medtech development is because of the infrastructure we have in artificial intelligence. Montreal is a hub in AI,” including such firms as Imagia (which got its own $3 million in support from the BioMed Propulsion program) and cardiac health firm Corstem. “Over the next 25 years there will be a wave of medtech startups incorporating AI, algorithms and deep learning devices,” said Arless, “and Montreal will be at the center of it.”
Alexandre Le Bouthillier, COO and Cofounder of Imagia, told me that as he worked for three years to figure out how to apply AI to personalized medicine, it was clear there would be a shortage of talent globally, “but Montreal is an attractor of talent,” including five strong researchers who applied to work with him from Switzerland, Italy and the States. “We see that more and more,” he said. “People from Silicon Valley are moving here.” For the longest time, Canadian firms were best known for the price tags they commanded when acquired. And investors would ask them to relocate to Silicon Valley or Boston. “Now, when we meet investors south of the border,” said Le Bouthillier, “they don’t ask that anymore.”
Last year, the Canadian government announced an investment grant of up to $49 million in the Digital Health and Discovery Platform (DHDP), a network of partners led by Imagia and the Terry Fox Research Institute that seeks to establish a Canada-wide health data platform to accelerate the development of new and personalized treatments to help to find cures for diseases. With $108 million in case and $165 million in in-kind contributions, the network connects nearly 100 partners across Canada, including health care institutions; SMEs; universities and research foundations; and all four major AI research labs in Canada.
NIH Looks to Accelerate Digital
The U.S. federal government is getting into digital health innovation too. Before the pandemic became everyone’s top priority, The National Institutes of Health this spring launched a $1 million Technology Accelerator Challenge to spur the design and development of non-invasive, handheld, digital technologies to detect, diagnose and guide therapies for diseases with high global and public health impact. The challenge is focused on sickle cell disease, malaria and anemia and is led by NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB). The Bill & Melinda Gates Foundation is cooperating with NIH to help accelerate the transformation of design concepts into products for low-resource settings.
“Bioengineers are pioneering the development of cutting-edge, cost-effective, mobile and point-of-care technologies,” said NIBIB Director Bruce Tromberg, Ph.D. “While this challenge is not constrained to any specific technology, the inspiration for it comes from the widespread availability of mobile phones and the potential for mobile phone-linked sensor technologies to non-invasively detect changes in the blood and blood vessels associated with these treatable diseases.
“New diagnostic tools could address a major burden of disease in low- and middle-income country settings,” said the Gates Foundation’s Dan Wattendorf, director of Innovative Technology Solutions, Global Health. “Handheld, low-cost tools can bring testing out of a laboratory and to the point of need.”
In April, the need was COVID-19 testing. Digital health tech may be coming to the rescue there too. Menlo Park, California–based BillionToOne announced it had developed a highly accurate and cost-effective novel COVID-19 test protocol, unlocking more than 1 million testing capacity per day in the U.S. alone. Test reagents were to be available by the end of April, pending manufacturing of kits and emergency use authorization from the FDA.
The key to its development? Bioinformatics. BillionToOne’s proprietary machine learning algorithms unlock testing capacity by allowing genome sequencing to expand by more than 30 times the capacity of current methods.
“BillionToOne’s molecular counter improves the resolution of cell-free DNA testing by over a thousand-fold, meaning we can detect disorders other tests can’t,” said Oguzhan Atay, PhD, co-founder and CEO of BillionToOne, when the firm closed its $15 million Series A funding round last year, led by Hummingbird Ventures and NeoTribe Ventures with participation from Y Combinator, Civilization Ventures, Fifty Years, 500 Startups Istanbul and HOF Capital. “It’s the difference in finding a haystack verses the needle in the haystack. The result is an increase in accuracy at a much more granular level.”
“We are excited to join BillionToOne,” said Kittu Kolluri, co-founder and managing director of NeoTribe, “and be a part of its growth as they revolutionize the clinical utility of genomic sequencing.”

