Time was when Malaysian medical and health products were based primarily on the natural resources of the country – for example, medical gloves made of rubber. Leadership in those categories is still strong: Hartalega Holdings is constructing a world-class manufacturing complex in Selangor with six factories and 72 high-tech production lines. All told, the $580-million project, which was recognized by Site Selection as a Top Deal of 2012, will employ up to 5,000 workers when complete in 2021, and produce more than 28 billion gloves a year.
Supermax Corp. Berhad manufactures gloves in Malaysia that meet the highest global standards, and is building its 10th and 11th factories. And Top Glove is in the midst of a $1-billion, 15-year expansion program that includes Top Glove Tower, a 23-story office building in Setia City, Shah Alam, Selangor, not far from the company’s 16 advanced manufacturing facilities in Meru, Klang. Top Glove’s investment has been recognized as an Entry Point Project under the Economic Transformation Programme by the Malaysian Government.
But while continuing the trend as the world’s leading producer and exporter of catheters and surgical and examination gloves, Malaysia is now diverting its focus to higher value-added medical devices products and the ecosystem surrounding this sector. And Malaysia’s business ecosystem supporting the sector is growing as fertile, diverse and full of life as the nation’s rainforests.
“MIDA believes that the ecosystem approach is the best way to retain investors, as this offers a stronger reason for companies to remain and grow in Malaysia,” says Y. Bhg. Datuk Noharuddin Nordin, CEO of the Malaysian Investment Development Authority (MIDA), noting the adoption of the approach applies to a number of industries. “There is a strong presence of established supporting industries conforming to world-class standards support the medical devices industry in Malaysia. The supporting industries capable of meeting the needs of the medical devices industry range from sterilization services, sterile medical packaging, electronic manufacturing services [EMS], precision engineering and tool and die making to contract molding and assembly and machinery fabrication in Malaysia.
“Companies in the medical devices ecosystem are encouraged to improve their competitiveness and capabilities to reap substantial economic benefits by moving up the value chain and propel them towards becoming global players in the future,” he says. “This will further attract more quality investments from both local and foreign sources and will also encourage innovation in new products. Such positive spin-offs are very much in line with the Government’s initiative in transforming Malaysia towards a high-income nation.”

MIDA CEO Y. Bhg. Datuk Noharuddin Nordin
Malaysia’s healthcare system already is second to none in ASEAN, and has sparked a medical tourism industry that welcomes visitors from around the globe. Meanwhile, the nation’s electronics industry heritage has lent its work force the requisite skills in quality management and precision that in turn lend themselves to such sectors as pharmaceuticals and medical devices.
Matt Szuhaj, director, strategy & operations for Deloitte Consulting LLP and head of the life sciences practice, says Malaysia may be lumped by some with other emerging markets when doing site selection analysis, but thanks to its stable business and regulatory environment and government support, deserves to be compared with such locations as Taiwan and South Korea.
“If you look at it from an R&D perspective, it’s very attractive,” he says. A lot of countries say they’d like to compete in the life sciences, he says, but Malaysia has “taken steps to actually play in it. At the heart of it is competing with Singapore. They have the natural resources and the people to do it, and now they have the tax scheme and regulatory environment to support it.”
The timing couldn’t be better. A recent survey by PricewaterhouseCoopers found that 55 percent of multinational companies and 62 percent of domestic companies believe that Asia, rather than North America and Europe, will be the focus market for life sciences and healthcare for the foreseeable future. Part of that focus is the medical device sector, which Frost & Sullivan sees growing in the Asia Pacific from just under $50 billion in revenue in 2012 to more than $106 billion by 2018. And with a combined population of 600 million and gross domestic product of US$1.8 trillion, the ASEAN market offers immense trade opportunities.
Szuhaj points out that Malaysia is not just an Asia Pacific play. “Because of the way they move these products [primarily by air], you can reach the global market. There’s not a logistics constraint. It’s more about reliability and security than about the cost to the supply chain.”
Adding to that sense of security is intellectual property protection. Szuhaj says IP concerns are not an issue that keeps companies from considering Malaysia. And the country continues to take steps to alleviate any concerns.
“The Government is very serious about it,” says Datuk Noharuddin. “We have improved our IP protection, especially industrial, and have set up a court to handle cases of infringement. For the medical device industry, this is extremely important.”
High Standards Are Standard
Companies in the medical device sector are feeling more secure in Malaysia with each passing year. The sector in 2011 was designated as a National Key Economic Area (NKEA) under the nation’s Economic Transformation Programme. And the Medical Device Act was enacted in 2012. With the enforcement of the Act commencing on July 1, 2013, all medical devices manufactured, imported or sold in Malaysia are required to be registered.
“We’re accustomed to meeting standards,” notes Datuk Noharuddin, referencing the country’s strong export markets in Europe, the US and Japan and its legacy expertise in precision electronics. “It’s not an issue for us.”
In late July, British Standard Institution (BSI), the business standards company, was registered by the Malaysian Medical Device Authority as one of the three Conformity Assessment Bodies (CAB) approved to date.
“This regulation will help facilitate international trade by reducing the regulatory barriers required for different regions and provide improved access to new technologies,” said Yap Liep Lin, Singapore and Malaysia managing director at BSI. “Manufacturers will benefit from the expertise and knowledge BSI can bring to getting products to market not only in Malaysia but around the world.”

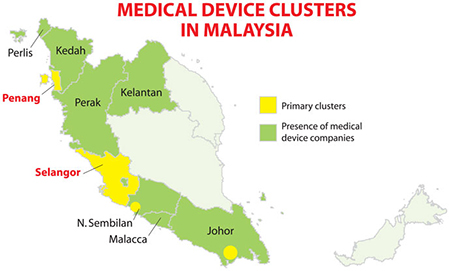
Known as home to one of the longest bridges in Asia, Penang is gaining further global renown for the breadth of its medical device cluster’s global connectivity.
This timing couldn’t be better either, as the Association of Southeast Asian Nations (ASEAN) looks to introduce a harmonized ASEAN Medical Device Directive (AMDD) that will improve the medical device importation, registration and distribution regulations in 10 member states in the region.
“With the implementation of the Medical Device Regulations, Malaysia will be recognized as a reliable producer of medical devices in the global market,” says Datuk Noharuddin of MIDA. “It helps Malaysia to attract foreign direct investment with increased market confidence and it will also enable more joint ventures between local and foreign companies.”
B. Braun’s Center of Excellence
A number of companies are setting up and expanding to serve regional and global markets.
B. Braun Medical Industries Sdn. Bhd. (BMI) is a subsidiary of B Braun Melsungen AG, Germany, one of the largest medical devices companies in the world. BMI has been in Penang since 1972. The site was the Group’s first manufacturing site in the Asia Pacific region, and remains one of the largest B. Braun manufacturing sites for the Group outside Germany.
According to the Association of Malaysian Medical Industries, medical device exports from Malaysia in 2012 totaled $3.9 billion, and are expected to grow by 6 percent in 2013.
Since its inception, BMI has invested more than RM2.3 billion (nearly US$702 million) in manufacturing facilities in Malaysia. A further investment of RM1.75 billion ($534 million) was announced in 2010, the largest ever in the medical devices industry in the country. The plant located in Penang is among the largest manufacturers of medical, surgical and pharmaceutical products not only in Malaysia, but in the region. B. Braun employs more than 6,500 people both at its plant in Penang and its sales and marketing office in Kuala Lumpur (established in 1980).
In 2006, the Penang plant was recognized as the Global Centre of Excellence (CoE) for Intravenous Access products including R&D and production technology. Products manufactured in B. Braun Medical Industries Penang are exported to the US, Japan, Germany and several major markets around the world.
“Malaysia is a good choice for manufacturing because of the skills and competency of its people,” says Dr. Juergen Schloesser, senior vice-president and head, Centre of Excellence – Intravenous Access. “Over the years of development, the local team has acquired the necessary know-how and has proven to be highly capable.”


B. Braun’s enthusiasm for Malaysia is reflected by its own employees’ enthusiasm for their workplace, which this year was voted among the Best Companies to Work For in Asia. The company’s 40 years of investing in the country include a 2010 announcement pledging $534 million toward a new complex (rendering pictured) in Penang.
Images courtesy of B. Braun
Just as helpful is the country’s location.

Dr. Juergen Schloesser, Senior Vice-President and Head Centre of Excellence – Intravenous Access of B. Braun
“Malaysia is strategically located within ASEAN region and at the same time along the major sea routes in Asia between the West and the Far East,” Dr. Schloesser notes. “The Regional Distribution Centre located in Penang helps us to serve the entire Asia-Pacific region efficiently.”
He says the three most important strategic advantages to having a Malaysia location in his company’s property portfolio are the skilled and highly motivated work force; multicultural and multilingual people with a good working knowledge of the English language even at the supervisory level; and the country’s stable political situation and economy.
Dr. Schloesser says the expansion announced in 2010 will cater to the growth of the company’s manufacturing output for the next 15 to 20 years, “allowing an increase of manufacturing capacity of an average 15 percent per annum while providing additional employment opportunities.”
He notes the engagement of economic development authorities and especially MIDA, noting that they are “business friendly and understand the needs of business. MIDA is staffed with highly competent people who are there to facilitate should any issue or problem arise.”
Onto Something Big
A decade ago, TH Su, CEO of Straits Orthopaedics (Mfg) Sdn Bhd., was wishing to return to the working world after a short-lived retirement as a corporate lawyer, and trying to drum up some business involving Malaysian electronics and potential opportunities with China and the U.S. What he found instead was a “fascinating” 500-page report documenting the coming growth in the orthopaedic industry in the United States.
“I was pretty excited,” he says in an interview at MIDA’s headquarters in Kuala Lumpur. “This was a sunrise industry I was looking at.” Not long thereafter, despite naysayers, he was able to establish his first component supply relationship in the sector in 2002, promising and delivering a 40-percent savings. Today the sun may be approaching its zenith. Su’s company has grown from two small lots in Penang to 32, employs 310 people, and has annual revenues of over $17 million that he projects will reach $20 million this year. And Su is still excited, as his company continues to flourish and to branch out.
Points of Entry
Malaysia’s medical devices industry has been identified and placed under the healthcare sector, which is among the 12 National Key Economic Areas (NKEAs). The Healthcare NKEA includes seven medical devices-related Entry Point Projects (EPPs), which are projected to create RM17.12 billion (US$5.2 billion) in revenue and generate 86,000 jobs by 2020. The seven EPPs for medical devices are:
- Tap into the fast-growing IVD (in vitro diagnostics) market through academic/industry partnerships: Leverage research & patented technologies to set up Asian champion in neglected diseases prevalent in developing countries: TB, dengue, cholera in Africa, Asia and South America.
- Next generation of single-use devices: Move the value chain towards high value products on core SUD products & build showcase for MD industry – focus on catheters, woundcare and single-use components for instruments.
- Build strong contract manufacturing champions: Becoming contract manufacturer for high value orthopaedic products, e.g. screws and instruments for global MNC brands.
- Create Malaysian clinical devices champion: Creating an enabling environment for Malaysian entrepreneurs to innovate in clinical devices and create their own brands in bone graft and other orthopaedic implants.
- Orchestrate contract manufacturing supply chain: Become turnkey production orchestrator for MNCs – from component sourcing via supplier audit to packing and sterilisation and logistics; provide in-house quality assurance.
- Re-manufacture medical equipment: Attract MNC to establish authorized medical equipment refurbishment facility in Malaysia for CT scanners, MRI and molecular imaging.
- Medical furniture and hardware cluster: Develop a medical furniture and hardware cluster, with a two-pronged strategy: focusing on home care in developed markets, while riding the wave of infrastructure build-up for hospital furniture in emerging countries.
“In the past few years, I came to the realization that what I was offering was only a small part of what orthopaedics was all about,” he says, noting the sector’s main segments of trauma, reconstructive and spine. Primary machining was the firm’s sole offer, with things such as reconstructive hips and knees outside its capabilities. What the company and his country needed, Su realized, was the capability to offer plating and forging, building “upon the capabilities and the ecosystem of contract manufacturing.”
Straits is poised to join forces with a European forging leader and jump from only being able to do 40 percent of potential trauma products to 100 percent. At the same time, Su is teaming with an Italian firm in a joint venture to do coatings, which gets the company into the replacement hip market. And Sima Medical Sdn Bhd – a collaboration among Straits, Chinese company Naton Medical Group and Malaysian firm OSA Technology Sdn Bhd – is also launching in Penang with a $33-million investment.
Su knows the big US companies are under intense cost pressure. He also knows he can offer prices 30 percent below their costs, while also offering an entry point to the emerging markets of the Asia Pacific, where growth is outpacing established markets.
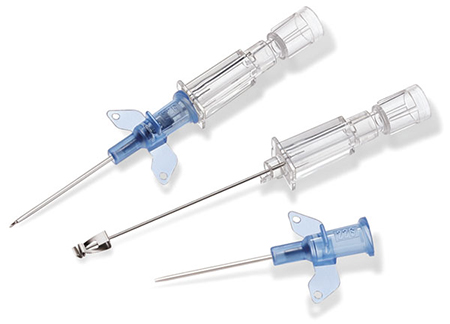
A sampling of the products manufactured by B. Braun in Malaysia
“The most exciting place right now is Asia Pacific,” he says. “There is a huge population base, rising industrial activity and a rising standard of living. Reconstructive is a big thing. With rising standards of living and health care, people are living longer. So there is this dynamic shift. The leading names are coming to Asia Pacific and looking at it as the market for future leadership in the world in orthopaedics.”
Su says competition from China and India only makes his firm leaner and better, and he knows from past experience that he can beat those competitors. His first new venture, ABio Orthopaedics, went into operation in August with a $17-million facility investment. It will initially deal in hips, knees and other joints, including extremities, with the possibility of expanding into what Su calls the underestimated market of dental implants.
“The combination of ABio and Straits will synergize the other solutions of casting and coating,” he says. “That synergy will allow Straits and ABio to tackle 63 percent of the orthopaedics revenue being generated year on year” by 2016. That is, 63 percent of an industry valued at $43 billion.
The Value Creators
“The idea is to make Malaysia visible on the orthopaedics map,” Su explains. Altogether there will be five companies offering the full array of machining, casting, coating and forging capabilities.

TH Su is CEO of Straits Orthopaedics
Central to continuing the success will be maintaining the science at the core of the business, and that means not only adherence, but devotion, to high standards such as those of the US Food and Drug Administration, one of several global authorities whose input helped create the high standards now part of the nation’s new medical device regulations.
“The Medical Device Act is very good for Malaysia,” says Su.
Education and training are also accorded high priority in national development under Malaysia’s five-year development plans. Su says the strong science programs at Malaysian universities mean it’s easy for him to find talent in such fields as mechanical, biotech and chemical engineering. At the same time, he trains those high-level graduates in the fundamentals of precision manufacturing, and looks to his operators for top-notch execution and insights.
“Inspection does not create quality,” he says. “My operators have come up with beautiful stuff – they are the people who create value,” he says.
Top Shelf
Malaysia’s recent rankings include:
- 10th most attractive destination for FDI (A.T. Kearney’s FDI Confidence Index)
- 12th in ease of doing business (World Bank Doing Business 2013 Report)
- 14th most competitive economy in overall performance (IMD’s World Competitiveness Yearbook 2012)
- 25th most competitive nation in the world; 2nd highest-ranked Southeast Asian country after Singapore and 6th in Asia after Singapore, Hong Kong, Japan, Taiwan and South Korea. (WEF’s Global Competitiveness Report 2012-2013)
Su’s company attained Pioneer Status Incentive from MIDA. “That allowed me to really invest everything to increase the capacity of my business,” he says. “The second thing MIDA gave me was the approval for expatriate posts for scarce skillset required in the orthopaedic area.”
In Malaysia there are currently 15 education/training institutions which offer different levels of medical device-related qualifications ranging from certificate to doctorate level. There are also higher learning institutions which provide short courses and up-skilling programs. In January 2013, MIDA established an Industry Talent Management Division to address and service investors’ human capital needs.
In December 2012, the Malaysian Government launched the Graduate Employability Blueprint for 2012-2017 focusing on boosting the level of graduate marketability as well as fulfilling the needs of the professional and skilled manpower towards national development. An initiative by the Northern Corridor Economic Region, Penang Skill Development Centre and Association of Malaysian Medical Industries has resulted in a program specifically tailored for the medical devices industry. In November 2012,to support the growth of the medical devices sector, Info Kinetics Sdn Bhd and Clinical Trial Centre for Medical Devices of Yeungnam University Hospital from South Korea agreed to formally establish cooperation and collaboration in medical device testing and clinical trials, e.g. in-vitro biocompatibility testing according to ISO 10993, toxicology studies, leaching studies and medical devices clinical trials according to ISO 14155. This will lay the foundation for the first fully structured medical device clinical trials center in the Northern Corridor Economic Region (Koridor Utara), long the most active area for the industry.
Su’s new venture in ABio will receive its own support from MIDA, but Su praises the agency for more than its incentives. He likes its devotion to what the country can be by 2020.
“To have a vision is one thing – are you prepared to walk the talk?” he says. “Having seen the way they dedicate themselves and the passion they bring to the table, they really inspire me. I’m not flattering them. I say it from my heart. MIDA energizes me.”
Asked if his new capabilities stretch beyond orthopaedics, his smile broadens.
“If I am not unlucky – because I’ve been lucky so far – the solutions I bring to this country like forging and casting have applications across industries like aerospace, oil and gas and high-end automotive parts,” he says. “MIDA is very interested in bringing in these capabilities.”
Startup Mentality
Vigilenz Medical Devices Sdn Bhd is a manufacturer of surgical sutures, hernia mesh, disinfectant products and other medical disposables led by S. Choudhury, managing director. Like Su, he started his company because of the entrepreneurial urge, and the desire to see the sector reach a new level of sophistication and diversification. Even stronger was his sense of national pride.
“I looked at the landscape in Malaysia,” he says. “I didn’t see too many local companies. It was something I felt passionate about. Why not? We have the talent.”
Incentives for Devices
Malaysia prefers that investors make their decisions based on their assessment of the fundamentals in Malaysia and not just the incentives. But the country steps up with a healthy array of incentives nevertheless, including income tax exemptions and investment tax allowances for manufacturing and high-tech companies and strategic projects; various allowances and exemptions for different categories of R&D; and exemptions from import duty on raw materials/components and from import duty and sales tax on machinery/equipment, spare parts and consumables. For more details, visit www.mida.gov.my.
His firm set up shop in 2002 and gradually accumulated the international standards and quality certifications that gave it credibility. In 2007 Vigilenz became the first Malaysian company to receive the Class III CE Marking for medical devices.Having gone from sutures to wound wash, the company next expanded into wound dressing, and is now looking at entering the anti-microbial marketplace.
The company employs close to 90 on property first leased and then purchased from Penang Development Corp. This year Vigilenz invested in another plot of land, and looks to be in operation there by the end of 2014.
Choudhury thinks Malaysia sets up perfectly as a clinical research base for this niche, with English-speaking professionals, and a work force familiar with high production standards.
He also highlights what he sees as a unique strength of Malaysia: the diversity of sterilization options, with five different facilities either online or about to be, offering gamma, steam, electron beam and ETO (ethylene oxide) methods. “This is huge,” he says. “No one in the whole of ASEAN has what we have.”

S. Choudhury, Managing Director, Vigilenz
Choudhury says MIDA’s life sciences division first visited his company in 2004, and Vigilenz stayed on MIDA’s radar as small-to-medium enterprises grew more central to MIDA’s strategy. He hopes to see Malaysian educational institutions start to become incubators for entrepreneurial groupings of firms.
“When entrepreneurial thinking comes in, a lot of things will change, and people will start to flock in,” he says, noting his own recent hiring of a top polymer science student at a comfortable salary for both employer and employee. “Think of the amount of things you can do with people specialized in polymer science,” he says. “The whole world is moving towards that. Biopolymers and the materials sciences are huge.”
At some point, he says, a satellite operation elsewhere in the Asia Pacific is a possibility. But wherever that satellite might land, he says, “This will always be the headquarters of my company.”
That kind of talk is music to the ears of Malaysians, whether they’re government officials or citizens or both.
Healthy Climate
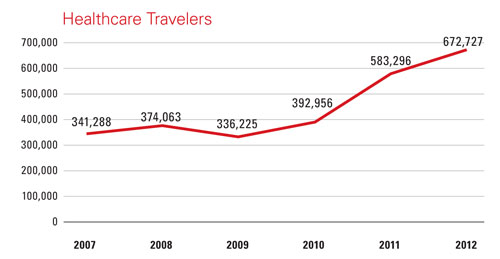
While Malaysia positions itself to increase its exports of medical devices, there is one strong trend angling to keep those devices in-country until they’re used: medical tourism. According to the Malaysia Healthcare Travel Council (MHTC), an agency under Malaysia’s Ministry of Health, heart bypass surgery in Malaysia can cost as much as 90 percent less than in the United States. In 2012, 671,000 medical tourists were served at 72 private hospitals registered with MHTC. And some 12,000 US citizens alone come to the country every year for treatments. More generally, Malaysia recorded 25 million tourist arrivals in 2012, good for a No. 10 ranking by the UN World Tourism organization.
Malaysia ranked 2nd in the “2013 Health Care Survey: The Best Havens for Quality Care Overseas” by International Living. And Prince Court Medical Center (Malaysia) was named No. 1 among the World’s Best Hospitals for Medical Tourists 2013 by Medical Travel Quality Alliance. MIDA CEO Y. Bhg. Datuk Noharuddin Nordin says Malaysia is poised for the widely observed convergence of the healthcare and life sciences sectors.
“Both clusters need each other,” he says. “Malaysia is positioning itself as a hub for the healthcare industry. That lends itself well to supporting the development of the medical devices industry as well. In the early days, medical tourism was the only objective. And there’s another major activity that’s taking place – the rapid growth of clinical trials in Malaysia, which take advantage of medical institutions as well as universities.”
“The timing is perfect” for Malaysia, says MIDA’s Datuk Noharuddin. “We want to leverage on the presence of these anchor companies to develop the ecosystem. We have the [ASEAN] economic community around the corner by 2015, and we have our own Economic Transformation Programme. There’s growing interest in Asia – it seems to be the only place that’s growing at the moment.”
The leverage points also include an awareness that goes beyond borders.
“We are pursuing investment by applying this ecosystem concept,” he says, “but it doesn’t mean it needs to be within Malaysian borders. You have to acknowledge some may be in neighboring countries. We can position ourselves as a market within the broader ecosystem.
“But of course,” he says, “we have a naturally strong ecosystem within Malaysia.”
This Investment Profile was prepared under the auspices of the Malaysian Investment Development Authority. For more information, visit www.mida.gov.my.
