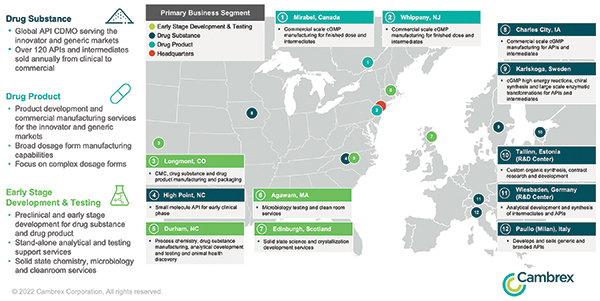
Forty years after its founding, New Jersey–based drug substance manufacturer and contract development and manufacturing organization (CDMO) Cambrex is investing in facilities in such locations as Charles City, Iowa; Tallinn, Estonia; High Point, North Carolina; Karlskoga, Sweden; and Milan, Italy.
Cambrex, based in East Rutherford, New Jersey, has invested around $300 million over the past five years in its network of flexible small, mid-sized and large-scale manufacturing facilities as it grows its team of over 2,200 employees.
The company’s career site in January showed 130 open positions in North America and eight at its sites in mainland Europe and the UK as it announced progress on over $100 million of investments across its global manufacturing network of 12 locations.
I recently talked to Thomas Loewald, Cambrex CEO by email about how the company manages its site portfolio and global location decision-making in this ultra-competitive sector.
Your company newsroom is peppered with facility expansion announcements from Iowa to Estonia over the past two years, starting just before you came on as CEO. Describe the composition of your team that makes these strategic location decisions, and how they go about their work.
Thomas Loewald: Our Executive Leadership Team runs a long-range strategic planning process each spring. Coordinated by our chief strategy officer, this process begins with a look at market trends within the CDMO industry using a combination of internal and external data sources, which we distill down to the relevant themes for each of our businesses. From there, each business unit leader is responsible for developing the organic growth plan for their business, taking into account our current customer/product portfolio, installed capacity base, and expected new customer/product wins resulting from their team’s commercial efforts. We expect each business unit leader to bring forward a plan that meets or exceeds the market growth rate and identifies the top two to three organic growth drivers for their business.

“Piramal’s investment is tremendous news for Scotland’s life sciences sector, one of the largest life sciences clusters in Europe, and is creating new, high-quality jobs that are crucial to our economic recovery.”
— Adrian Gillespie, Chief Executive of Scottish Enterprise, on the Indian company’s investment at its Grangemouth site
One of the outputs of that process is a rough outline for where we think we will need to add capacity over time, what form that capacity should take, and a high-level cost estimate. Then it becomes a matter of monitoring the market and ensuring we pull the trigger soon enough on investments to avoid capacity constraints as we grow. Because we plan our capacity over a multi-year time horizon, the business case is well developed by the time we need to approve the spend formally.
We are fortunate to be part of a dynamic and growing industry, and we have existing facilities that lend themselves to bolt-on capacity expansions with attractive economic returns. That’s why we have been so aggressive about expansion projects over the last few years, and the market has supported that growth.
Walk me through a couple of these project decisions that stand out in your mind in terms of overcoming challenges, key turning points or above-and-beyond support.
Loewald: In the first half of 2020, as the COVID pandemic swept the world, we saw a surge in demand for COVID-related therapies that stretched our operational teams to the limit. Fortunately, the long-range planning process I described earlier left us in a position where we had sufficient capacity to take on COVID-related production to support the urgent public health need while also continuing to support our existing customers and products.
At the same time, the surge in demand put us over where we like to operate in terms of capacity utilization in large-scale API manufacturing, so we made the decision to kick off our next phase of expansion with a $50 million project in Charles City, Iowa. That expansion was already planned to occur within a couple of years, but we pulled it forward to ensure we could continue to take on new work for our customers. Now, two years later, the COVID-related demand surge is mostly behind us, and we’re filling that new capacity in Iowa with multiple new products, supporting the development pipelines of our customers in the process. (The Iowa site’s expansion is the sixth facility expansion investment made there by the company since 2008.— Ed.)
As Cambrex facilities evolve, what facility design or workspace innovations are you implementing to better meet the needs of employees and customers?
Loewald: With any investment, we aim to deliver improvements that benefit both our customers and employees. Lean lab concepts are one design feature that meets that criterion by making labs a more organized, convenient place for our employees to operate and drives efficiency that enables us to serve our customers better.
Electronic lab information management systems — LIMS for short — are another example of a tool we are investing in across all our facilities. By implementing LIMS, we are pushing critical lab data into an electronic system and out of paper notebooks, creating a more robust quality management system for our customers and a more efficient way of working for our employees.
Finally, during COVID, we implemented a new technology called Realwear to enable customers to experience being in one of our facilities without needing to physically travel. Realwear includes a head-mounted camera that allows our current and prospective clients to tour our facilities and see the manufacturing and testing services for themselves in real time from anywhere in the world.
The company’s facilities are often near university and technical school campuses such as the Tallinn University of Technology. What do you look for from higher education institutions in terms of R&D partnerships, internships or skills training, and which institutions are doing a good job of delivering?
Loewald: Scientific expertise is at the center of the experience we deliver for our customers, so recruiting and developing that scientific talent is among our most critical tasks as a company. Accessibility of talent in the local labor market, whether it be from the industry or local universities, is among the criteria we use when considering where to make our next investments. Local universities with excellent chemistry and chemical engineering departments are ideal for the type of talent we hire in our highly skilled laboratory and manufacturing roles. In addition to the Tallinn University of Technology you mentioned, we have had success recruiting from the University of Iowa and North Carolina State University, to name a few examples.
What would you identify as your top challenges: Talent development? Speed to market? Supply chain and equipment provision? Regulations?
Loewald: At our core, we are a service business, and therefore our most important challenge is delivering a uniformly excellent customer experience across our network of 12 facilities. Whether we provide a salt or polymorph screen in Edinburgh, a clinical API batch in High Point, or commercial production at one of our large-scale API or finished dose facilities, our customers should experience the highest level of quality, service, and depth of scientific expertise. Delivering a consistent experience across a global network requires constant coordination, IT systems that enable collaboration, and a passion for commercial excellence. We recognize that our customers have options when deciding which CDMO to partner with on their molecule. High quality standards, speed and reasonable cost are table stakes, but a truly excellent customer experience provides an opportunity for differentiation.

Seqirus is moving vaccine R&D to a new site in Waltham, Massachusetts.
Image courtesy of Seqirus
What continues to make New Jersey the right place to be headquartered?
Loewald: Our New Jersey headquarters serves as a landing point for our management team, which is a global team that generally works virtually from the U.S. and Europe. Easy to access by various forms of transportation, it sits at the center of the East Coast life science clusters that range from Boston to North Carolina. From a customer point of view, the state is host to 13 of the top global pharma companies, and in 2021, 40% of all new FDA approvals came from a company that had a foothold in that state. New Jersey also has the highest number of biopharma manufacturing facilities in the U.S. Finally, our office’s proximity to New York’s financial services industry has proven convenient when we’ve needed to tap into capital markets for growth over the years.
In which regions of the world will Cambrex look to grow next?
Loewald: Today our network spans North America and Europe, and we intend to continue adding capacity and new technologies in those markets as our primary focus. Those are the geographies where the vast majority of the pharma R&D pipeline resides, and our customers have demonstrated a clear bias toward high-quality, Western suppliers over time. While an expansion to new markets is possible, our core focus will continue to be North America and Europe.
Project Roundup
Major biopharma projects abound early in 2022:
Eli Lilly and Company in January announced it would invest more than $500 million in a new 300-job biologics active ingredient manufacturing facility in Limerick, Ireland, and more than $1 billion in a new manufacturing site in Concord, North Carolina, that will create nearly 600 new jobs making injectable products and devices. In 2020, Lilly made its first manufacturing investment in North Carolina with a $470 million investment at a former GSK site in Research Triangle Park. Lilly said it “selected Concord because of the manufacturing technology experience of the local labor force; its proximity to universities with strong science, technology, engineering and math (STEM) programs; and its access to major transportation infrastructure.”
The company first set foot in Ireland more than 40 years ago, in 1978, with the purchase of a farm in the West Cork countryside of Dunderrow, near Kinsale.
“Since our initial investment, Lilly has continued to grow our footprint in Ireland in part because of supportive government policies that value life science discoveries and innovation,” the company said. “With this new investment in Limerick, Lilly will expand our manufacturing network for biologic active ingredients, support demand for existing products and play a key role in bringing our robust pipeline, including our promising neurodegeneration portfolio, to people around the world.” The company also said further manufacturing investments would be forthcoming.
Seqirus, a business of Australia-based CSL Limited that is focused on influenza prevention, in February announced an investment in a new R&D facility in Waltham, Massachusetts. The custom-built facility consists of approximately 140,000 sq. ft. with 54,000 sq. ft. of lab space, with the ability to house about 300 full-time employees. The new site is expected to be fully operational by mid-2022 as work transfers from an existing site in Cambridge, Massachusetts.
“Our current facility in Cambridge houses about 110 employees,” Seqirus spokesperson Maria Tortoreto tells Site Selection. “Our investment in the new R&D site in Waltham aims to create over 200 jobs, allowing for 300+ employees to work on-site. All ongoing programs currently taking place in Cambridge will fully transition to the Waltham facility in the coming months, enabling the new site to become our central R&D hub. Once this transition occurs, we don’t anticipate any further work at the Cambridge site.”
“With more than 1,700 scientists around the world, CSL is making important R&D investments in leading biotechnology locations,” the company said, including Melbourne, Australia; Bern, Switzerland; Marburg, Germany; King of Prussia, Pennsylvania; Pasadena, California; and Amsterdam, Netherlands.
FUJIFILM Corp. in January agreed to acquire for $100 million a cell therapy manufacturing facility form Atara Biotherapeutics in Thousand Oaks, California. CDMO FUJIFILM Diosynth Biotechnologies will operate the site. “At closing, Fujifilm plans to offer positions to approximately 140 current highly-skilled manufacturing and quality staff at the site,” the company said, noting that the new facility will expand its manufacturing footprint to the West Coast and complement its existing locations supporting the advanced therapy market in College Station, Texas; Watertown, Massachusetts; and its recently announced BioCampus in the United Kingdom. The CDMO overall has existing locations in Teesside, UK; Research Triangle Park, North Carolina; College Station, Texas; and Hillerød, Denmark, and is currently building new facilities in Watertown and Holly Springs, North Carolina.
India-based Piramal Pharma Limited’s Pharma Solutions CDMO in January announced the expansion of its Antibody-Drug Conjugate (ADC) capabilities at the Grangemouth facility in Scotland and the investment in new Active Pharmaceutical Ingredient (API) infrastructure at the Morpeth facility in England. The £45 million (US$??) project at Grangemouth is backed by a grant from Scottish Enterprise, and will add up to 50 new jobs at a site that already employs 200. The £10 million (US$ million) investment in API operations at Morpeth, backed by a similarly-sized grant from the UK government, will help protect around 400 jobs.
Alcami, a North Carolina–based pharmaceutical contract development and manufacturing organization (CDMO), announced in January a $10 million investment to expand its lab operation in Durham, North Carolina, adding to a portfolio of 675,000 sq. ft. at sites across the U.S.
BioCentriq announced construction was complete on a GMP-ready clinical manufacturing site in South Brunswick, New Jersey, next to its existing pilot plant.
Molecular Stethoscope, a precision medicine biotech company founded at Scripps in San Diego, established its new corporate HQ and expanded its R&D labs in Verily Life Sciences lab ecosystem in South San Francisco. “We are excited and energized to establish our new corporate headquarters and expand our R&D laboratories in South San Francisco, which positions us to attract and retain the best talent, grow our clinical and academic collaborations, expand our commercial partnerships, and drive the development of our clinical-grade cf-mRNA liquid biopsy pipeline infrastructure to support our roadmap of products and services,” said Guillermo Elias, Ph.D., CEO at Molecular Stethoscope.