T |
he medical technology industry – medtech for short – is very much in vogue in Europe. For one thing, corporate investors crawled out of the dot-com rubble and sought sanctuary alongside what they thought would be the next golden goose. But this sector is more complex than the financial world thought, being multi-faceted, capital intensive, alliance-dependent, political and highly regulated.
The medtech sector can be carved into three segments: life sciences (including the booming biotechnology sector), pharmaceuticals, and medical devices and equipment. The global market for medical technology – that is all three segments combined — was worth around US$580 billion in 2001. The US is very much the medtech giant, representing over half the world market, which is growing at around 10 percent per annum and will continue to do so for the next 10 years. But China is the real medtech darling, outpacing all the other significant medtech markets, although not yet in the same league as the US or Europe
The European League
So who are the players in the European medtech league, and in what do they specialize? A new report just published by Oxford Intelligence, Investment Strategies & European Benchmarking in Life Sciences, Pharmaceuticals, Medical Devices and Equipment (The MedTech Report), has some answers.
According to the report, the UK, Germany and Ireland are the most popular European destinations for international direct investment projects in the medtech sector. US companies are the dominant international investors, accounting for 62 percent of international direct investment projects worldwide during 2001. Looking forward, 92 percent of the North American companies interviewed by Oxford Intelligence had plans for future international operations in the next two years; Germany and the UK are the preferred countries.
The most often mentioned European locations for future medtech manufacturing facilities are the UK and Germany, followed by Ireland and then the Czech Republic. For research and development facilities, Germany beat the UK slightly in the rankings, but also often quoted were Scandinavia (particularly Denmark and Sweden), and also France, Spain and Switzerland. According to the report, Western Europe has a major opportunity to be a hub for the higher value-add generation of bio-pharmaceuticals that will come to market en masse over the next five to 10 years.
Sticking Points in Europe
Despite the high regard in which US medtech corporates hold Europe, there are some sticking points. For a start, manufacturing in Western Europe is expensive, although less so in Southern Europe, and low labor cost regions will become increasingly significant for medtech manufacturing. The Czech Republic, Hungary and Poland are the prime candidates for this opportunity, although there is increasing competition from the Far East and India. According to The MedTech Report: “If Eastern European Governments can ensure strict respect for intellectual property, this will give them a critical competitive advantage over the Far East and India.”
Clinical trials will become increasingly global and, rather morbidly, there are not enough potential patients in the West to fulfil medtech companies’ requirements. Eastern Europe, Latin America and the Far East will play an increasing role in future clinical trials. For Eastern Europe to continue to compete, it has to make available to incoming contract research organizations facilities with high-speed IT and communications services.
Research and development in Europe will only be attractive to US companies if the location in question can provide access to skilled labour, high quality academic facilities and a significant student base. According to The MedTech Report: “Many regions across Europe have universities with excellent life sciences faculties, yet do not have significant medtech industry clusters. The fact is that international companies’ first choice would be to locate R&D facilities in the well-established European R&D clusters, such as Cambridge in the UK, and Munich, because the skills are already there. This means that other regions need to develop their indigenous medtech clusters.”
Government Agencies’ Medtech Role
Also surveyed by Oxford Intelligence were the European economic development agencies, which were benchmarked according to their ability to attract investment from medtech companies into their regions. A total of 125 European regions were scrutinized and narrowed down to 21 key regions by surveying European regional and national economic development agencies, interviewing senior industry executives, evaluating agency Web sites and desk research. The analysis provides a comparison of key European regions across life sciences, pharmaceuticals and medical devices.
A number of benchmarking parameters were chosen, reflecting key location drivers in international investment projects. The regions were ranked according to: the level of foreign direct investment they had achieved; the regional corporate infrastructure (companies, labor pool and start-up activity); corporate taxation levels, transport links, research and development indicators (value, capacity, and output); and economic development agency proactiveness indicators. Weights were applied to emphasize the relative importance of the different criteria.
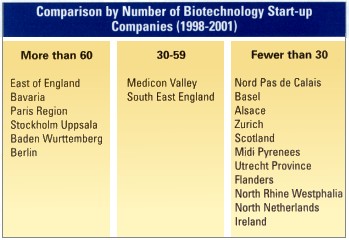
Seven regions scored highest across the benchmarking parameters, led by Baden Wurttemberg (South West Germany), Southeast England (including the key university city of Oxford), Bavaria (especially Munich) and the Paris region. Berlin, North Rhine Westphalia and Stockholm Uppsala completed the top seven regions.
The second tier of agencies included the R&D intensive Swiss powerhouses: Basel, Geneva and Zurich; the UK’s East of England (especially Cambridge), Greater London; Scotland; Nord Pas de Calais (Northern France); and Medicon Valley, the Danish/Swedish Scandinavian biotech hot-spot. The third-tier key regions — – in Belgium, France, Ireland and the Netherlands — all have particular strengths that can be compelling reasons for selecting them for a European operation.
Ireland, for example, has been targeting medical devices, equipment and pharmaceutical manufacturing in recent years as a key investment sector, and this is clearly reflected in its top 20 status. The country’s two key attributes have been its low corporate taxation rates and the proactiveness of the national agency, which ensured that Ireland’s awareness among the corporates questioned was the highest of all European regions. Its drawbacks are related to the limited size of its workforce and its relatively poor transportation links (both domestically and internationally). The single biggest challenge facing Ireland is the establishment of a research base to compete with the best in Europe.
Government Agency Wake-up Call
North American medtech corporates are investing heavily in European operations, but only 20 percent of North American companies interviewed (the majority of which had established new European operations over the last four years) had used economic development agencies to assist them with their most recent European investment projects. This is unfortunate, since over 90 percent of the companies questioned had plans to establish future international operations within the next two years.
The report concludes that the key challenge for European economic development agencies is the need to adopt a more flexible approach to inward investment promotion, one that is less driven by judging success on the volume of direct job creation. This is particularly the case for the biotechnology industry, where cross-border alliances and strategic partnering are the primary mode of globalisation, innovation and production.
According to Dr. Henry Loewendahl, Senior Consultant at PriceWaterhouseCoopers–Plant Location International: “It is no longer so much about attracting an R&D center or manufacturing plant (although this is still important), but rather about developing an environment conductive to alliances and providing the support services to facilitate cross-border alliances. This requires a whole new approach to investment promotion.”