What does the world of photography have to do with the drug business? It’s all about having the right chemistry.
Before digital imaging took over, photography was a chemical-intensive business (often leaving behind toxic traces as well as beautiful photographs). Today the photo industry giants have shifted that chemical expertise to active pharmaceutical ingredients (APIs) and other biopharma applications. That shift is gaining further impetus from the pandemic-induced push to reshore to the U.S. some of the biopharma supply chain.
Fujifilm early this year announced plans to invest $2 billion in large-scale cell culture production “in the vicinity” of its U.S. sites. At first blush, that would seem to indicate choosing one of its two major sites in College Station, Texas, or Research Triangle Park, North Carolina.
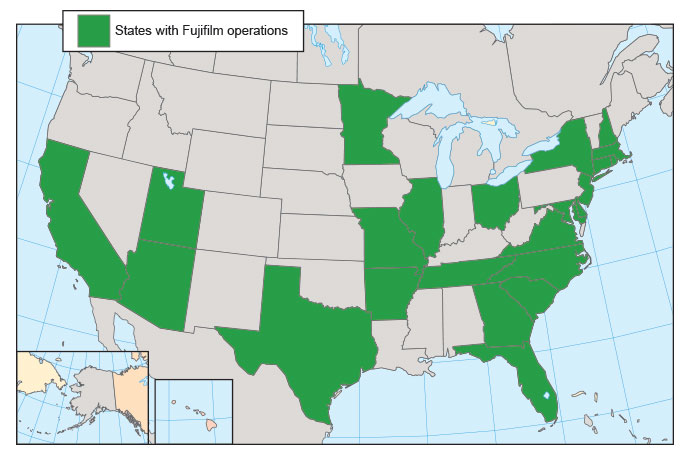
But in a February phone conversation with Martin Meeson, CEO, Fujifilm Diosynth Biotechnologies, I learned the site selection for this investment encompasses all Fujifilm sites in the U.S. (see map).
“That was a broad ask, given we’re in 25 states,” Meeson says, but a reasonable one. “You could argue that we got down to just half. Leveraging our capabilities and expanding out from where we are gives you a base to work from, gives you a nucleus of people, and you can start recruiting people much more easily.”
Has the deliberate choice to announce the $2 billion intent without having chosen a final location meant states have come courting?
“Yes, we are getting contacted by many locations and having discussions with many states within the U.S.,” says Meeson from company offices located on the borderline between Cary and Morrisville in Greater Raleigh. “It’s really just about the way we transact our business. We try to be open about what we do … there’s only so much we can do in secret. Ourselves and the board felt it was best to be open about our desire to be in the U.S., and it sends a good and strong message to our customer base.”
It’s just one project among many for the Japanese giant, whose investments within and outside of Diosynth tracked by Site Selection over the past several years include expansions in both College Station and Raleigh; Billingham on Teesside in the UK; Tilburg, Netherlands; Madison, Wisconsin; Tokyo and Hillerød, Denmark, where the company last fall pledged to invest $928 million after acquiring the site for $890 million from Biogen. Even as that transaction was unfolding, the COVID-19 Therapeutics Accelerator, an initiative launched by the Bill & Melinda Gates Foundation, already had reserved manufacturing capacity at the Fujifilm site. Ground was broken for the new Denmark expansion early this month.
In January, just before the announcement of the looming mega-investment, Fujifilm Diosynth announced a $40 million investment in a new process development and manufacturing facility for viral vectors and advanced therapies in the growing Boston-area life sciences magnet of Watertown, Massachusetts. The company secured $76 million in financing and signed a lease for a 40,000-sq.-ft. site in at The Arsenal on the Charles, owned and operated by Alexandria Real Estate Equities, Inc. Fujifilm and Alexandria are two of the partners in the new Massachusetts Center for Advanced Biological Innovation and Manufacturing (CABIM) located at The Arsenal. Other partners include Cytiva (formerly part of GE Healthcare Life Sciences), Harvard and MIT, working with collaborators such as Beth Israel Deaconess Medical Center, Boston Children’s Hospital, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Massachusetts General Hospital and MilliporeSigma.
Contract manufacturing services for early phase clinical trials are expected to begin at the new Fujifilm site in fall 2023, two years before the yet-to-be-determined location for the $2 billion investment is slated to launch.
Transformation Right Before Your Eyes
But back up a minute: How do we trace the threads between photography and life-changing advances in biochemistry? A helpful explainer sent to me by Fujifilm brings the picture to life as clearly as a print in a darkroom tray:
“At the turn of the current century, Fujifilm’s ‘photo related’ product lines – including cameras and film — were more than half of its revenue,” says the company. In March 2020 the company reported that figure has been reshaped to less than 20% as part of the company’s diversification strategy.”
Fujifilm was already predicting digitalization by the beginning of the 1980s, and by the early 2000s had launched “a rigorous evaluation to determine what ways the company could thrive, if analog film production were to go away. Fujifilm leveraged its centers of excellence, including its proprietary core assets — nanotechnologies in chemistry, mechanics, optics, electronics and imaging — breakthroughs that its film and camera legacies had given the company.”
Fujifilm’s expertise in its main product — color photographic films — proved particularly fruitful in the development of businesses in biopharmaceuticals, regenerative medicine, display materials and cosmetics. “Color film requires very advanced technologies for evenly applying as many as 20 different photosensitive layers of different functions into film measuring just 20 microns,” the company explains, “then precisely arranging in each of the layers over 100 types of compounds — including color-sensing and color-producing particles. The disciplines of process control, quality assurance, and microscopic analysis from Fujifilm’s color film advances have proved to be very transferable to the current state of microbiological and pharmaceutical research and manufacturing.”
That includes the high-precision world of regenerative medicine R&D, where “Fujifilm now is one of the only companies in the world that possesses the tissue engineering triad necessary for regenerative medicine — cells, scaffolds, and cell culture medium/cytokine.”
Like a highly engineered therapy itself, today’s Fujifilm grew from being able to steer legacy assets and capabilities toward new targets.
Kodak’s Moment
In another well scrutinized process — involving a presidential executive order and a potential large federally backed loan — Kodak is moving further into the API business at its home base in Rochester, New York.
Last July, on the very day that former President Trump was extolling the work of Fujifilm in manufacturing a crucial pharmaceutical ingredient in North Carolina, the federal government announced support for the conversion of Eastman Kodak’s plants in Rochester, New York, for active pharmaceutical ingredient (API) manufacturing. A $765 million loan to Eastman Kodak Company from the U.S. International Development Finance Corporation (DFC) was to support the launch of Kodak Pharmaceuticals, a new arm of the company that will produce critical pharmaceutical components. The project is expected to create 300 jobs in Rochester and 50 in Minnesota, and it marked the first use of new authority delegated by Trump’s executive order that enabled the DFC and the U.S. Department of Defense to collaborate in support of the domestic response to COVID-19 under the Defense Production Act (DPA). “By leveraging our vast infrastructure, deep expertise in chemicals manufacturing, and heritage of innovation and quality, Kodak will play a critical role in the return of a reliable American pharmaceutical supply chain,” said Kodak Executive Chairman Jim Continenza.

Site Selection has been following Kodak and its properties’ various incarnations for decades, including insights from longtime Kodak corporate real estate leader H. Bruce Russell in 1997; updates on the company’s redevelopment of property it’s had since 1880; a spring 2012 look at the company’s role in Rochester’s resilience; and this 2016 Investment Profile about a younger generation and agribusiness making things happen at Eastman Business Park, where the company’s API business will grow.
As reported by FiercePharma and others over the winter, the DFC loan to Kodak was suspended after numerous investigations into how the deal came together, although a report by Anthony Zakel at the Office of the Inspector General absolved the DFC of wrongdoing. A separate investigation commissioned by the company’s board cleared Kodak leaders of insider trading accusations, as Kodak shares had shot up following the original announcement that leaked before all DFC ducks were in a row.
Continenza in October pledged that Kodak was entering the API business with or without the DFC loan. That was confirmed by a Kodak spokesperson in response to a question from Site Selection. In March, a series of transactions provided the company with up to $310 million of incremental cash to invest in growth opportunities.
“Over the past two years, we have taken a number of significant steps to strengthen our financial position,” said Jim Continenza. “Financing secured through Kennedy Lewis and investments made by Grand Oaks Capital and funds managed by Southeastern Asset Management represent the next step in our strategy for returning the company to growth and help position us to invest in expanding our core businesses in print and advanced materials and chemicals.”
Replication and De-Risking
Fujifilm is well down the investment path, and ready for more. CEO Martin Meeson credits the “great teams around me” for the company’s success. “Sometimes you need to expand those teams,” he says, “as we are doing at the moment with a significant number of announcements in the past nine months.”
Meeson says the investment decisions in Denmark and Massachusetts, like all investment decisions, were based on demand evaluation. “That always has to be the place you start,” he says. “We get line of sight on demand, line of sight with the way the market is growing,” then evaluate the capabilities of the company and its supply chain partners against that. The Denmark decision came first because it leverages the capabilities of that site as the company looks to build its ability to do large-scale antibody manufacturing.
“But as you make that first decision,” he says, “it springs into your mind what the next will be. The conversation around that has been ongoing for several months. There was a lot of evaluation,” which led to a slightly more difficult decision. “Do you go again in Denmark? Or do you start to push out into other locations,” he asks. “Obviously the U.S. is the largest market for this particular class of molecules we make, so it was a natural choice. As we look as well to replicate what we have in Denmark, it’s attractive to the customer base to have the dual locations. It de-risks the supply chain to have the same capability in multiple locations.

“The other investments we’ve made at a smaller scale but similar in nature,” he says, “investments in microbial capacity, extending out in the UK to match demand, investments in advanced therapies areas. The initial idea was to expand the offering we had in Texas, but what we’re looking at now is a more regionalized strategy around therapies.”
Viral vectors is an example. An initial investment came to the UK in order to have a presence for customers in the European continental zone. Now the high density of developers and a mature hospital and medical network in Massachusetts have begotten the Watertown investment.
“I’ll take a risk and say it’s quite unique in the world,” he says of the life sciences ecosystem in Greater Boston. “There are some pockets in Europe and the UK in particular, and some clusters are building up in other areas that are similar in density. We deal with a large number of customers in Massachusetts, and work with them to commercialize products in these facilities. But there is such an opportunity there, from initial development capacity to transfer to commercialization and ultimately first in human products. We’re still with a view that commercialization would be in the main hub in Texas, and other places in the world as we expand.”
Coming into Focus
In essence it seems, the development center and its large cadre of scientists in Texas possesses critical mass for the company, but the density of organizations in Denmark and Massachusetts is exerting its own magnetic pull. Other salient factors in the site selection include ease of access to the space by clients (as more in-person work comes back after the pandemic); the cost of creating and maintaining infrastructure; time and ease of further development; and the sustainability of the location.
By sustainability, Meeson clarifies, he’s not just talking about soundness in terms of the environment and employee well-being, but in terms of being built to last.
“You need to see five to 10 years out to make sure you have the line of sight for infrastructure that has what you need,” he says. As for the corporate sustainability profile, he says, it’s important to determine how the operating environment will help Fujifilm attain its governance goals. “This is becoming an increasingly important factor, and can be a real differentiator.”
And in the context of the pending project decision, talent is one of the most important factors in the world of advanced therapies.
“Not just the talent, but the pipeline of talent,” Meeson says, noting the strong academic network in the Boston-Cambridge region, “as we have in a number of locations in which we operate. This is about developing a larger pool. One thing we’ve been focused on for the past two decades is we are intrinsically embedded in this talent development.”
In North Carolina, the company was a founding member of the NCBiotech industry organization. Similar support has come in Texas via Texas A&M University, among others. The company and the Texas A&M University System Center for Innovation in Advanced Development & Manufacturing (CIADM) in January announced that production had begun in Texas on two different COVID-19 vaccine candidates with support from the U.S. government to meet Operation Warp Speed goals. The company works with Teesside University and others in the UK, and has recently gotten involved with the Manufacturing Academy of Denmark.
“That was just a continuation of the push to make sure we’re at the forefront of promoting this as an industry sector,” he says. “Where else do you get a chance to go to work and create medicines that change people’s lives? It’s a really interesting sector and has a wide variety of disciplines we need: scientists, engineers, logistical roles and straight-up manufacturing roles,” supported by a strong company apprenticeship and vocational skills program.
Presumably that talent pipeline will already be strong wherever the company lands with its $2 billion decision. No doubt the project itself will make such a pipeline even more robust.
“It would be so much easier if one just popped out of the pile,” Meeson jokes about the site selection decision. “I was not naïve enough to think that would happen. We are starting to narrow it down and getting people to lock in some of what they’re offering with a very finalized version of what the package they are looking to assist us with looks like. As you can imagine, this is a significant investment, and it will require quite a lot of briefing. There are a few layers to go through to make sure that briefing is done properly and the appropriate authorizations are sought by the correct people in the Fujifilm Diosynth and Fujifilm organizations.”
The aim is to narrow it down by the end of the first quarter, says Meeson.

