Two new studies from Pugatch Consilium consultancy shed light on global regional competitiveness in the biopharma arena. And Canada is a surprisingly low-ranked performer.
The first study, “Measuring the Global Biomedical Pulse: The Biopharmaceutical Investment & Competitiveness (BCI) Survey,” was commissioned by US pharma industry lobbying group PhRMA. So it will come as no surprise that the U.S. and European economies continue to top the global charts in terms of attractiveness for biopharmaceutical investment.
“Notwithstanding low costs and considerable potential, emerging markets still come in at the bottom,” says Pugatch of the 15 nations studied. “Canada is also found to be surprisingly low on the list compared to developed economies, mainly due to challenging intellectual property policies.”
The BCI, a global survey-based index of economies’ biomedical competiveness, polls 350 biopharmaceutical executives of top-ranking multinational companies operating in 16 economies — who serve as ambassadors to their companies of the investment attractiveness of each market. By gauging their confidence in a given market and translating it into a quantitative score, the BCI enables a unique and highly relevant snapshot of economies’ biomedical competitiveness.
Top performers in the BCI — the US, UK, Switzerland and Ireland — all score above 80 percent of the total possible score and place at the top of the sample in most of the seven major categories of the survey, which range from the ability to leverage R&D and manufacturing capabilities to the regulatory, IP and market environment. “All four boast excellent and effective scientific research systems, regulatory and intellectual property (IP) frameworks that meet the highest international standards and relatively supportive market access environments.
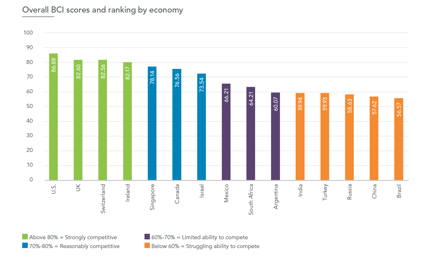
Markets falling into the bottom quarter of the sample — the BRIC economies plus Turkey — score less than 60 out of 100. “Though each market has its own specific challenges, common threads exist across all five,” says Pugatch, “particularly in the areas of regulatory quality and efficiency, ability to secure a fair price and protection of biopharmaceutical IP rights.”s
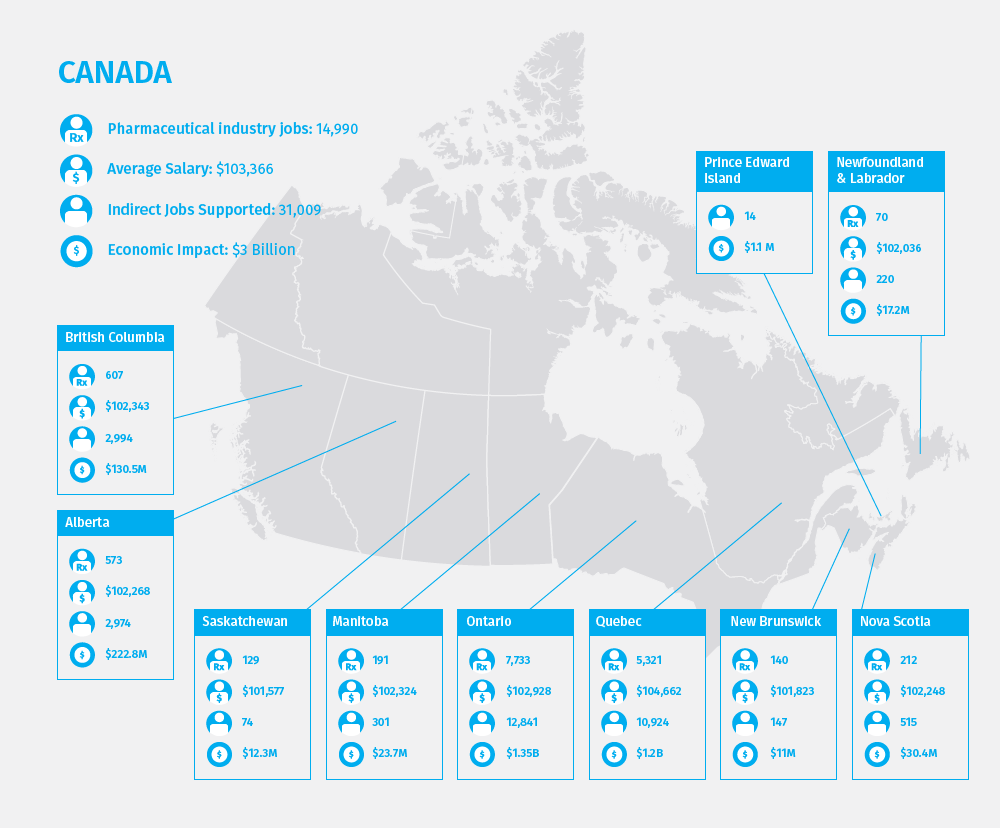
According to the firm, Canada has the least attractive environment among the developed economies, scoring 20 percent below the top developed market. “Local executives classified both Canada and Japan’s pricing and reimbursement systems as being particularly stringent, rating the market access environments in both economies more on par with emerging economies than developed ones,” says the report. Rx&D, Canada’s pharmaceutical industry association, did not respond to a request for a statement on the finding. The map below, however, illustrates the sector’s influence and relatively robust health across each Canadian province:
“Whether a high- or middle-income economy, the message is the same,” said Prof. Meir Pugatch, Managing Director at Pugatch Consilium. “In economies where policies affecting the biopharmaceutical environment present substantial challenges, local executives also rank these economies as having a lower ability to compete for investment from their companies. Nevertheless, in markets that have taken major steps to improve elements of the policy environment — such as Singapore and Israel, which have made significant strides in IP and regulatory standards in recent years — executives display much greater confidence, with these economies averaging at least 75 percent of the total possible BCI score.”
Did We Mention Regulation?
The second study, “Building the Bioeconomy 2015,” available in its entirety on the Pugatch Consilium website, devotes 65 of its 102 pages to analysis of the biotech ecosystems in 13 countries: Brazil, China, India, Korea, Malaysia, Mexico, Russia, Singapore, South Africa, Switzerland, Turkey, the UK and the United States. Malaysia, Mexico, South Africa, Turkey and the UK are newly added to the group since last year’s edition of the report. (See below for a full chart of the 15 markets’ strengths and weaknesses.)
The report features a new performance metric, the Biotech Policy Performance Measure. It also identifies seven enabling factors:
- Nurturing human capital: “A basic and fundamental building block for the biotech sector is the availability of high skilled and technically trained human capital.”
- Investing in physical and technological infrastructure for R&D: “R&D infrastructure and capacity is critical to fostering innovation and activity in high tech sectors including biotechnology.”
- Protection of intellectual property rights (IPRs): “IPRs such as patents and regulatory data protection are historically of real importance to the biotech and biopharmaceutical innovation process as they incentivize the development of new technologies and products. Maintaining a stable, efficient and predictable regulatory environment The regulatory and clinical environment in a given country or region plays a significant role in shaping incentives for innovation and establishing levels of quality and safety for biotech products.”
- Introducing technology transfer frameworks and enhancing public-private collaborations: “Technology transfer is an important mechanism for commercializing and transferring research from public and governmental bodies to private entities and private to private entities.”
- Providing for market and commercial incentives: “Market and commercial incentives can come through a number of different forms such as tax incentives and R&D credits for investments in plant, equipment and other R&D infrastructure. For the biopharmaceutical sector pricing and reimbursement systems for medicines and health technologies can have a profound impact on the incentives for biopharmaceutical innovation.”
- The existence of legal certainty and protecting the rule of law: “The general legal environment, including as it relates to the rule of law and the rule of law within a business context, is crucial to commercialization and business activities.”
The report continues to hammer home the importance of the regulatory framework, and is not afraid to offer up examples:
“In particular, internationally competitive processing times for clinical trials applications and market authorization applications for new drugs and technologies is critical,” says the report. “For example, two emerging markets with significant potential and high expectations in developing a biomedical and biopharmaceutical capacity, Brazil and Turkey, suffer from long regulatory delays. For example, in Brazil approval for clinical research needs to go through two separate government agencies and approval times can stretch to over one year compared to three months in the US and EU. Similarly, Turkey suffers from long delays in its market authorization process. Although Turkish regulators have stated that a submitted application must be processed within 210 days, surveys of manufacturers suggest that waiting times can exceed three years.
“This can be contrasted with Mexico,” the report continues, “where regulatory authorities have committed to and implemented reforms that cut the approval time for drugs already approved in the US, Canada, and EU from 360 days to 60 days.”
A Bioeconomy Ecosystem Snaptshot of 13 Nations
| Country | Success Stories | Stumbling Blocks |
|---|---|---|
| Brazil | • Government support for ag-bio and industrial biotechnology e.g. EMBRAPA and sugar-cane ethanol. • Brasil Maior initiative – focus on life sciences and need for improving human capital |
• Challenging IP environment • Biopharmaceutical P&R environment challenging strict pricing policies and local preferencing • Cumbersome tech transfer framework |
| China | • Significant investor in human capital and R&D infrastructure • High levels of IP creation through patenting (General and biotech) |
• Challenging regulatory environment for clinical trials and seed registration and commercialization • Strict reimbursement policies have limited the number of biological drugs available • Challenging IP environment |
| India | • Tradition of strong Government focus on biotech • Potential policy change by Modi Government – focus on innovation, improving IP standards |
• No comprehensive national tech transfer framework • Uncertainty over Government support for commercialization and registration of ag-bio products • IP environment: Section 3(d) and patentability requirements; no RDP; use of compulsory licenses and patent revocations for biopharmaceuticals |
| Korea | • High levels of R&D investment • Comprehensive tech transfer legal framework in place • Strong IP environment |
• No comercialized ag-bio products • Data requirements for pharmaceutical patent applications exceed international best practices • Strict pricing policies and limited reimbursement |
| Malaysia | • Generous high tech and biotech specific credits e.g. BioNexus • Relatively high level of technology transfer and patenting by palm oil PRO (Mlaysian Palm Oil Board) |
• RDP legally in place but limited in practical availability • Delays in marketing approval of biopharmaceuticals • P&R environment challenging – long formulary delays |
| Mexico | • Growing biopharmaceutical FDI – circa USD1 billion in 2012 • Cut in market approval processing times by COFEPRIS |
• Biopharmaceutical P&R environment challenging • Limited tech transfer framework in place • RDP available but unclear for large molecules |
| Russia | • High number of natural science PhDs • Generous R&D tax credits available |
• Limited commercial use of ag-bio products – regulatory not in place • Strict localization and P&R policies |
| Singapore | • World class biopharmaceutical R&D and manufacturing hub • High levels of clinical trials • Strong IP, regulatory and tech transfer environments • Generous R&D tax credits available |
• No commericalcultivation of ag-bio products • Limited industrial biotech |
| South Africa | • Strong tradition of ag-bio use and production • Generous R&D tax super deduction available • Technology transfer framework in place |
• Challenging life sciences IP environment • Limited biopharmaceutical R&D capacity • Long delays for pharmaceutical market authorization |
| Switzerland | • High levels of human capital • Leading global investor in biopharmaceutical R&D • Stong clinical trails environment |
• No commerical production of ag-bio products • GM foods in effect banner |
| Turkey | • Generous general R&D tax credits available 150% deduction • Growing number of life sciences graduates – 250% increase since 2000 |
• Challenging biopharmaceutical IP environment • Ag-bio R&D taking place but no commercialization • Strict P&R policies for biopharmaceuticals |
| UK | • Top life sciences universities in the world; Cambridge and Oxford ranked 3rd and 4th • High levels of clinical trials – per capita and total • Biopharmaceutical R&D almost 25% of total private sector R&D |
• EU regulations on ag-bio not conducive to wide-spread commercialization and use of ag-bio products |
| US | • Top life sciences universities in the world • World’s highest total of clinical trials • High total biopharmaceutical R&D • Biggest producer of ag-bio crops in the world • Leading producer of biofuels in the world |
• Uncertainties over patentability of basic biotech inventions e.g. 2013 Molecular Pathology v Myriad Genetics and 2012 Prometheus Laboratories, Inc. v Mayo Collaborative Services |

